Abstract
Background: An accurate assessment of kidney function is necessary to evaluate candidates seeking chimeric antigen receptor T-cell (CAR-T) therapy.(Jain, et al, 2019, Yakoub-Agha, et al, 2019) Additionally, kidney function quantification is essential to selecting the appropriate dose for fludarabine-based lymphodepleting chemotherapy prior to CAR-T cell infusion in order to optimize outcomes.(Hirayama, et al, 2019) Treatment decisions are currently guided by a glomerular filtration rate (GFR) that is estimated using serum creatinine. However, estimated GFR (eGFR) utilizing cystatin C-based equations has been suggested to overcome the known limitations of creatinine-based eGFR in patients with cancer.(Kellum, et al, 2012, Hersh, et al, 1986) The accuracy and precision of these eGFR assessment strategies in potential CAR-T therapy recipients has not been explored, and the preferred method for quantifying GFR in this patient population remains unknown.
Methods: This IRB-approved, single-center, observational study evaluated the performance of contemporary eGFR equations in any adult patient being evaluated for CAR-T therapy with a measured GFR (mGFR) by iothalamate clearance. Excluded individuals were those without a serum creatinine (SCr) and serum cystatin C (cysC) concentration performed within 14 days of the mGFR. Baseline characteristics and pertinent laboratory values were abstracted from the medical record. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations, including the most recent iterations that were developed without the race parameter.(Levey, et al, 2009, Inker, et al, 2012, Inker, et al, 2021) Bias, accuracy (percentage of estimates within 10% and 30% of mGFR), and precision (SD of the difference between the eGFR and the mGFR) were assessed. The prediction of mGFR by each eGFR equation was assessed with scatterplots.
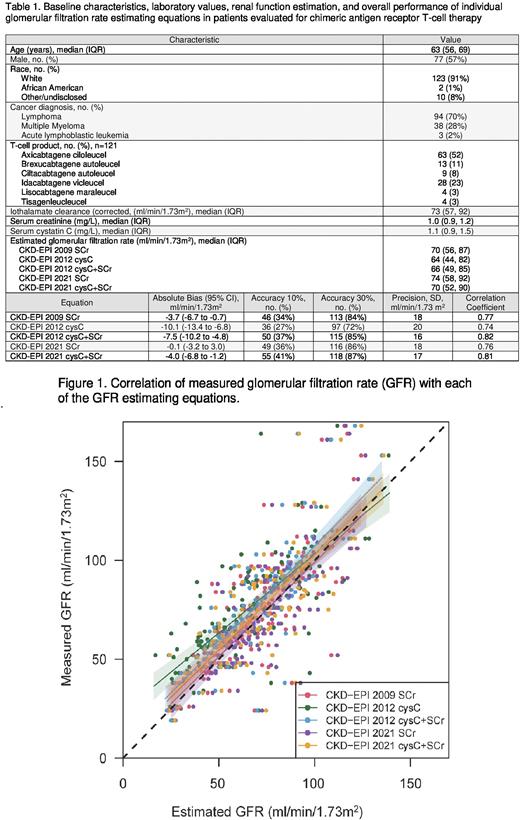
Results: The 135 patients that were included had a median age of 63 (IQR: 56, 69) years and 77 (57%) were male. Two patients (1%) were African American and 123 (91%) were White. Relapsed lymphoma was the most common cancer diagnosis (n=94, 70%), followed by multiple myeloma (n=38, 28%) and acute lymphoblastic leukemia (n=3, 2%). Median mGFR was 73 ml/min/1.73m2 (IQR: 57, 92) with mGFR >60 ml/ml/1.73m2 in 95 (70%) patients. Table 1 summarizes baseline characteristics, laboratory values, and eGFR. The average absolute bias ranged from -10.1 to -0.1 ml/min/1.73m2 indicating that all equations underestimated mGFR to some degree. CKD-EPI 2021 SCr and CKD-EPI 2021 cysC+SCr had the least bias at -0.1 ml/min/1.73m2 and -4.0 ml/min/1.73m2, respectively. CKD-EPI 2021 cysC+SCr had the greatest accuracy within 10% of GFR and within 30% of mGFR. Additional bias, precision, and accuracy details of each estimating equation are summarized in table 1. The CKD-EPI 2012 cysC+SCr and the CKD-EPI 2021 cysC+SCr equations most closely correlated with mGFR in all patients (r=0.82 and 0.81, respectively). Correlation of measured glomerular filtration rate (GFR) with each of the GFR estimating equations is depicted in figure 1.
Conclusion: CKD-EPI 2021 SCr and CKD-EPI 2021 cysC+SCr were the equations with the least bias. Contemporary eGFR equations had the greatest correlation with mGFR and seem acceptable when evaluating CAR-T therapy candidacy. Accuracy when dosing medications with a narrow therapeutic index, such as chemotherapy, is paramount, and all eGFR methods had limited accuracy within 10% of mGFR. Though the CKD-EPI 2021 cysC+SCr demonstrated the highest degree of accuracy within 10% of mGFR and within 30% of mGFR, iothalamate clearance should be the preferred method for kidney function assessment in patients seeking and receiving CAR-T therapy. Future research should investigate the impact of fludarabine dose selection on clinical outcomes of CAR-T recipients.
Disclosures
Ansell:SeaGen: Research Funding; Takeda: Research Funding; Bristol Myers Squibb: Research Funding; Regeneron: Research Funding; Affimed: Research Funding; Pfizer: Research Funding; ADC Therapeutics: Research Funding. Wang:Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Loxo@Lilly: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Eli Lilly and Company: Membership on an entity's Board of Directors or advisory committees; Genmab: Research Funding; MorphoSys: Research Funding; Genentech: Research Funding; Novartis: Research Funding; InnoCare: Membership on an entity's Board of Directors or advisory committees, Research Funding. Shah:Astellas: Research Funding; Celgene: Research Funding; Marker Therapeutics: Research Funding. Kapoor:Sanofi: Honoraria, Research Funding; X4 Pharmaceuticals: Honoraria; Regeneron: Research Funding; Amgen: Research Funding; Ichnos: Research Funding; Loxo: Research Funding; Karyopharma: Research Funding; BMS: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Takeda: Research Funding; Casma: Honoraria; Pharmacyclics: Honoraria; Imedex: Honoraria; GSK: Honoraria; Cellectar: Honoraria; Oncopeptides: Honoraria. Kumar:AbbVie,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive,: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE,: Research Funding; MedImmune/Astra Zeneca,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck,: Research Funding; Novartis,: Research Funding; Roche: Research Funding; Sanofi: Research Funding; Oncopeptides: Other: Independent review committee. Dingli:Bristol Myers Squibb: Consultancy; GlaxoSmithKline: Consultancy; Janssen Pharmaceuticals: Consultancy; Novartis: Consultancy; Takeda Pharmaceuticals: Consultancy; Sanofi S.A.: Consultancy; Apellis Pharmaceuticals: Consultancy; Alexion Pharmaceuticals: Consultancy. Kourelis:Novartis: Research Funding. Leung:Takeda Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Lin:Bluebird Bio: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Vineti: Consultancy; Merck: Research Funding; Janssen: Consultancy, Research Funding; Novartis: Consultancy; Gamida Cell: Consultancy; Juno: Consultancy; Takeda: Research Funding; Sorrento: Consultancy; Legend: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal